Blog | 1/22/2026
How Organoids and Organ-on-Chip Models Are Reshaping Preclinical Development
By Daniela Hristova-Neeley, PhD, Darragh Kennedy, PhD, Anthony Hesser, PhD, and Nicholas McConnell, PhD
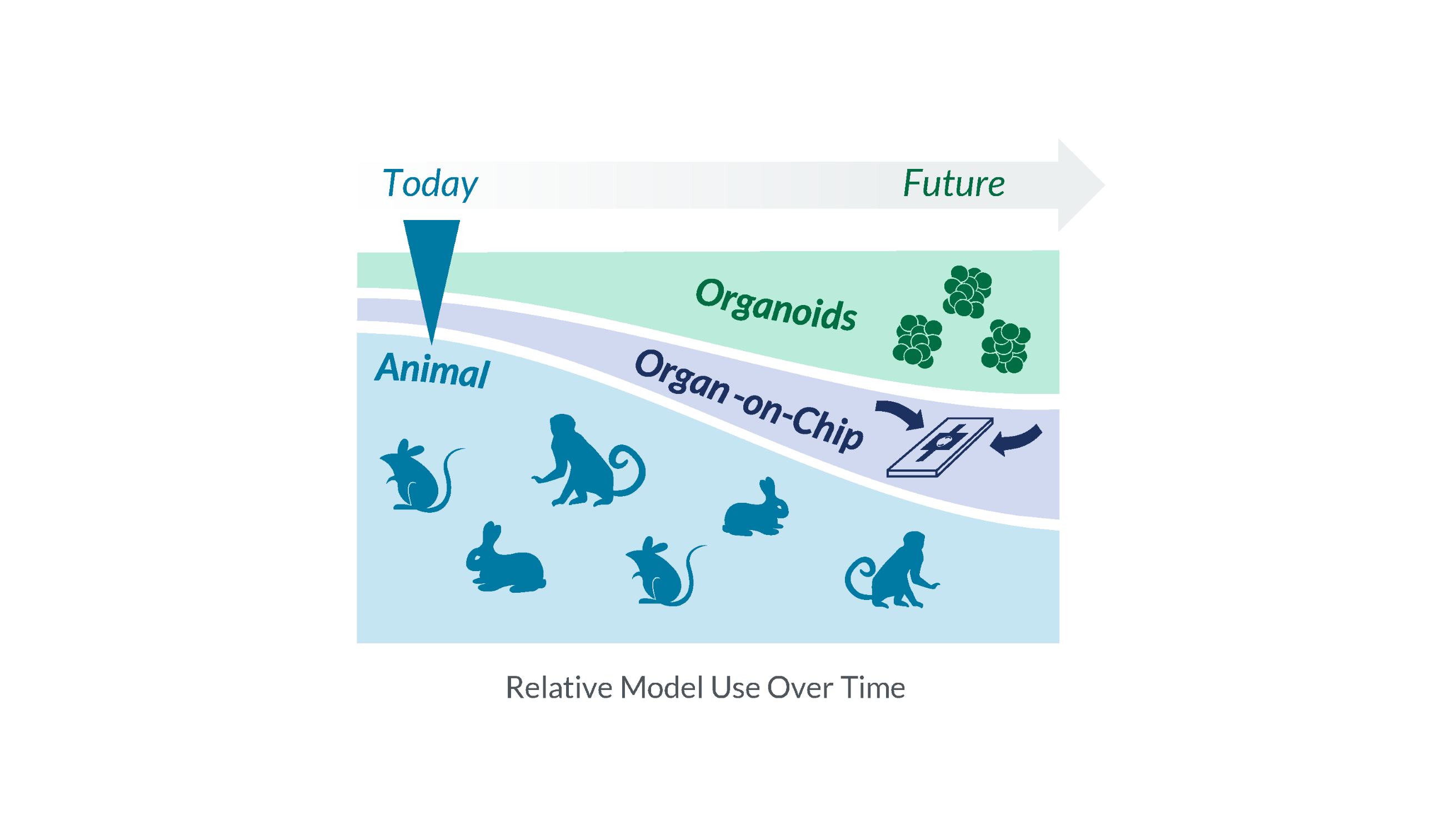
For decades, animal models have been the default approach to assess preclinical safety and efficacy of pharmaceuticals. That default is now being challenged by advanced in vitro models that are attaining buy-in from regulators. The current shift reflects a convergence of regulatory direction, capital investment, and operational scaling of New Approach Methodologies (NAMs) within a compressed time horizon.
This shift has potential for immediate consequences for how quickly preclinical programs advance, how risk is identified, and how capital is deployed. Organizations that view NAM adoption narrowly as a regulatory obligation will move reactively; those that treat it as a tool to shorten development cycles, reduce late-stage attrition, and differentiate their offerings will capture disproportionate value.
For tools, platforms, and preclinical CROs, this is an opportunity for growth.
1. Timing & Regulatory Precedent: Why the Shift Is Happening Now
Momentum behind organoids and organ-on-chip technologies has been building for more than a decade. NIH funding and scientific publications on organoids and organ-on-chip platforms have increased sharply, reflecting a rapidly maturing evidence base. The FDA has similarly gained momentum, shifting from signaling interest in animal testing replacement to setting direction.
In April 2025, the FDA released a roadmap articulating a clear ambition: make animal studies the exception rather than the rule within 3-5 years, beginning with monoclonal antibodies and expanding to additional drug classes. Recent guidance explicitly encourages the use of NAMs, including organoids, organ-on-chip platforms, and AI-enabled toxicity models in IND submissions. These approaches are no longer framed as optional innovation but increasingly positioned as potential sources of human-relevant evidence.
Milestones are already being achieved. In September 2024, Emulate’s Liver-Chip became the first organ-on-chip technology accepted into the FDA’s Innovative Science and Technology Approaches for New Drugs (ISTAND) program, a formal FDA qualification pathway through which novel drug development tools are evaluated and recognized for defined regulatory use cases, including drug-induced liver injury. Industry response has been swift. For example, Merck’s acquisition of HUB Organoids Holding B.V. and partnerships such as Emulate with Johnson & Johnson underscore accelerating validation. In parallel, AI players are entering the ecosystem, with Inductive Bio securing $21M from ARPA-H to develop toxicity models with Amgen.
Will animal testing disappear by 2028? Unlikely. But in specific use cases, such as hepatotoxicity and monoclonal antibody immunogenicity, NAMs are already becoming a decision-making tool. Our expectation is a hybrid model over the next 5-10 years in which drug developers will use NAMs to reduce the number of animals used in preclinical research.
2. What’s Needed for Wider Adoption
While the vision is clear, widespread replacement requires progress across several dimensions, many of which directly implicate tools and service providers:
- Regulatory Qualification & Standardization
Beyond liver models, additional organ systems (e.g., kidney, lung, gut) must enter formal qualification pathways such as FDA ISTAND. Harmonized protocols and QC metrics across vendors will be critical. - Scalability & Reproducibility
To support broader adoption, NAM platforms need to be standardized and the workflows to generate and analyze the models need to be automated to promote consistency across teams, sites, and sponsors. Players such as STEMCELL Technologies and InSphero are already investing in automated, scalable organoid and chip manufacturing workflows. - Comprehensive Validation
Single-site success may not be sufficient. A July 2025 multicenter study demonstrated that organoids derived from 184 patients across 17 tumor types mirrored real-world therapy responses, but regulators will likely require larger, multi-site datasets. Dedicated funding streams, including the NIH’s Office of Research Innovation, Validation, and Application (ORIVA), a public-private initiative focused on supporting large scale validation studies for organoid and other NAM technologies, may play an important role in generating the multi-site evidence required for adoption. - Integration with PK/PD & Immune Modeling
To more fully inform development decisions, organoid and organ-on-chip systems will need to be increasingly integrated with pharmacokinetic and pharmacodynamic (PK/PD) modeling, enabling, safety and efficacy signals to be integrated in the context of dose and exposure. - Regulatory Confidence
Reviewer training and clear guidance documents matter. FDA workshops in 2025 focused on reducing animal testing and NAM adoption represent an important foundation, but further specificity will determine the pace of uptake.
3. A Practical Adoption Timeline
Adoption of organoids and organ-on-chip models will unfold gradually rather than all at once. In the near term, these models are likely going to be used alongside traditional approaches, particularly in areas such as liver safety and other select questions for biologic drugs as researchers build experience and regulatory comfort. Over time, as qualification efforts advance and validation data accumulates, these models may supplement and replace animal studies for specific, well-defined questions, with hybrid strategies becoming more common. In 5+ years, continued standardization and regulatory confidence should support broader use of these model systems with animal studies increasingly limited to more exceptional use cases.
4. Implications for Tools, Automation Providers, and Preclinical CROs
As expectations for NAMs shift toward human-relevant data to support INDs, demand will increasingly favor tools and service companies that deliver reproducibility, scale, and regulatory credibility. Sponsors are likely to reallocate some preclinical spending from traditional animal studies to NAMs to support faster, more predictive decision-making.
Automation is becoming a critical enabler. Providers equipped with advanced liquid handling and imaging are better positioned to deliver standardized NAM workflows at scale. For example, Hamilton has published automated histology workflows for 3D models, while MIMETAS leverages Tecan’s imaging platforms to improve reproducibility in organoid formation.
For preclinical CROs, this shift to using more organoids and organ-on-chip solutions opens a new premium service layer: NAM-enabled preclinical development, where value is driven by insight and decision support, not just execution.
5. Several Strategies Tools and Services Companies Could Consider
For organoid and organ-on-chip platform developers, automation vendors, and preclinical CROs providing preclinical research services, the moment represents a rare opportunity to shape both standards and spend.
a) Anchor in Regulatory Qualification Pathways
- As regulators look to promote the use of NAMs, adoption can be facilitated by designing NAM studies with regulator review in mind, not just proof-of-concept
- Positioning platform and service offerings as submission-ready
- Using participation in programs such as FDA ISTAND as a signal of platform durability and legitimacy
b) Productize, Don’t Customize
As adoption scales, pharma will increasingly demand reproducible solutions over bespoke pilot models. To meet this demand, tool and service providers should:
- Standardize protocols, quality control metrics, and reporting formats
- Offer solutions targeted to specific R&D questions (e.g., liver safety, immunogenicity)
- Offer custom solutions that build upon a standardized core solution
c) Develop Automation-Enabled Workflows
Automation provides scalability, but also supports reproducibility that tool provides can promote with:
- Co-development of validated workflows with automation partners
- Published end-to-end protocols from sample prep through analytics
- Positioning of automation as a risk-reduction and compliance enabler
d) Lead or Orchestrate Multi-Site Validation Consortia
Regulator confidence is built from reproducibility across multiple sites. To build this confidence, tool provides can:
- Generate datasets linking NAM outputs to clinical outcomes
- Convene consortia spanning academia, CROs, and pharma
- Use these consortia to set de facto standards for specific applications
The shift away from animal testing is structural. As regulators, pharmaceutical sponsors, and capital align, research will consolidate around platforms that are standardized, automated, and regulator-aligned. For tools and services companies, the opportunity is not just to participate, but to define the infrastructure of next-generation preclinical development.
If these shifts resonate with your organization, we would welcome a discussion on how tools and services companies are positioning themselves as NAMs move from emerging solutions to standard practice. We are happy to explore how these dynamics apply to your specific goals.
For further discussion, you can reach us at: dhristova-neeley@healthadvances.com.

